Atoms are the building blocks of everything around us, including the human body. In order for atoms to form molecules—the units that make up all matter—they must first become ions. A positive or negative charge is added to or subtracted from an atom to create an ion. We refer to this process as ionization. So, let us know about what element forms an ion with an electronic configuration of ar and a –2 charge.
Ions are important because they are what allow atoms to form bonds with other atoms. These bonds are critical for everything from the structure of DNA to the function of muscles. In order for atoms to form these bonds, they must first be able to interact with each other—and that’s where ions come in. By becoming ions, atoms are able to interact with each other and form the molecules that make up all matter.
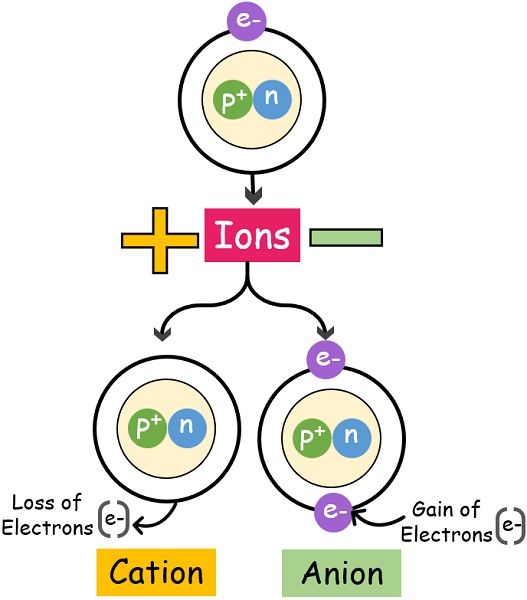
There are two types of ions: cations and anions.
An atom with one or more lost electrons and a positive charge is known as a cation. An atom that has gained one or more electrons is known as an anion and, as a result, has a negative charge. The number of electrons that an atom loses or gains determines the magnitude of its charge. For example, if an atom loses two electrons, it will have a charge of +2.
Types of Ions
Ions can be either monatomic or polyatomic. A monatomic ion is an ion that consists of only one atom. An ion with more than one atom is referred to as a polyatomic ion. For instance, the NH4+ ion, which is made up of a link between one nitrogen atom and four hydrogen atoms, is a polyatomic ion.

The electronic configuration of an ion determines its charge. The electronic configuration of an atom is the arrangement of electrons around the nucleus. The electronic configuration of an ion can be determined by looking at the periodic table. Each element has a unique electronic configuration, which corresponds to its position on the periodic table.
For example, the element sodium has an electronic configuration of Ar3d10 4s1. This means that it has 18 electrons surrounding its nucleus—two in the innermost energy level (1s orbital), eight in the next energy level (2s and 2px orbitals), and eight in the third energy level (3s, 3px, and 3py orbitals). When sodium forms an ion, it losses one electron from its outermost energy level (4s orbital). This gives it a charge of +1.
So, an ion is an atom that has either gained or lost electrons, resulting in a net charge.
Conclusion:
Ions are important because they allow atoms to interact with each other and form bonds—without these bonds, there would be no DNA, no muscle function, and no matter how we know it! Ions can be either monatomic or polyatomic, and their electronic configurations determine their charges. You can learn more about ions by studying chemistry or by reading about atomic physics!